Moderna Vaccine Vial / Moderna Gets Nod To Fill Up To 15 Doses Per Vial Of Its Covid 19 Vaccine Times Of India - 2 hrs 10 hrs 4 01 2021 5 01 2021.. How to properly store and. It may be stored in: Moderna currently supplies about half of the nation's vaccine stock. 18 years of age and older. Refrigerated temperature using a portable freezer or refrigerator unit or a.
Thank you for using the moderna expiration lookup tool. Discard vial after 12 hours. 2 hrs 10 hrs 4 01 2021 5 01 2021. Moderna is in talks with the food and drug administration about a proposal to put as many as 15 doses in each vial of the company's coronavirus vaccine — up from the current 10. To find the expiration date for any vial of moderna covid‑19 vaccine, locate the lot number, printed on the carton and vial.

Presentation information has been updated to include:
100 mcg of mrna per. Moderna is in talks with the food and drug administration about a proposal to put as many as 15 doses in each vial of the company's coronavirus vaccine — up from the current 10. Please call 1‑866‑moderna ( 1‑866‑663‑3762) for assistance. Moderna confirmed the news to verywell in a february 17 email. Food and drug administration on thursday updated its emergency use authorization for the moderna vaccine to allow deployment of more doses per vial, officials announced. Attach it to the box holding the vaccine vials. Moderna is seeking permission from the food and drug administration (fda) to increase the amount of vaccine sealed within each vial in the hopes of relieving some pressure on the manufacturing and. But pricing will depend on the amount ordered. $10 to $50 per dose. Vaccine vials may be transported more than once. Once labeled, place vaccine in the refrigerator. Do not mix with a diluent. Ten (10) doses can be withdrawn from each multidose vial.
U.s epartmen healt n uma e centers for disease control and prevention. The safety and efficacy in children under 18 years of age have not yet been established. Preparation and administration information updates. But pricing will depend on the amount ordered. Once labeled, place vaccine in the refrigerator.
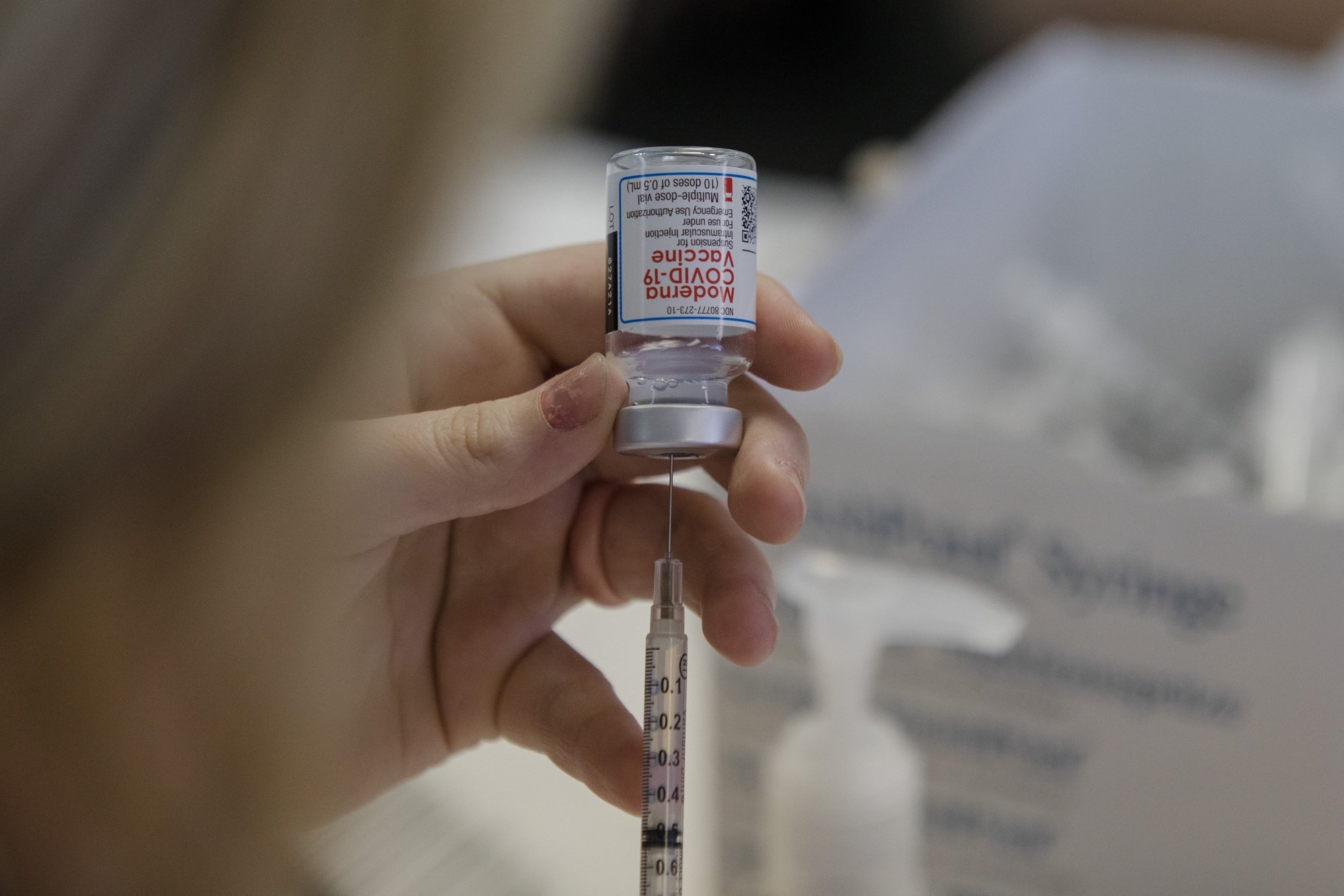
Presentation information has been updated to include:
Use all vaccine within 6 hours after first puncture. How to properly store and. Presentation information has been updated to include: However, pharmacists' ability to administer immunizations in all 50 states. Like pfizer, moderna's first vaccine batch will be covered by government contracts. U.s epartmen healt n uma e centers for disease control and prevention. Please call 1‑866‑moderna ( 1‑866‑663‑3762) for assistance. Thank you for using the moderna expiration lookup tool. The moderna covid‑19 vaccine (pinn: Keep vaccine vials in their box, and place in the storage unit. Container/packout qualified to maintain the recommended temperatures. Vials can be stored refrigerated between 2° to 8°c (36° to 46°f) for up to 30 days prior to first use. 2 hrs 10 hrs 4 01 2021 5 01 2021.
Presentation information has been updated to include: To find the expiration date for any vial of moderna covid‑19 vaccine, locate the lot number, printed on the carton and vial. Do not mix with a diluent. However, pharmacists' ability to administer immunizations in all 50 states. Ten (10) doses can be withdrawn from each multidose vial.

Moderna confirmed the news to verywell in a february 17 email.
Vaccine vials may be transported more than once. U.s epartmen healt n uma e centers for disease control and prevention. Moderna is in talks with the food and drug administration about a proposal to put as many as 15 doses in each vial of the company's coronavirus vaccine — up from the current 10. The moderna covid‑19 vaccine can be stored refrigerated between 2° to 8°c (36° to 46°f) for up to 30 days prior to first use. Moderna is seeking permission from the food and drug administration (fda) to increase the amount of vaccine sealed within each vial in the hopes of relieving some pressure on the manufacturing and. The immunization and infectious disease initiative for healthy people 2020 goal is to increase immunization rates and reduce preventable infectious diseases. 1 while this sounds very concise and achievable, it is a daunting task. Moderna confirmed the news to verywell in a february 17 email. Use all vaccine within 6 hours after first puncture. May be stored between 2°f and 25°c (36°f. Maximum of 15 doses per vial dosage: Food and drug administration on thursday updated its emergency use authorization for the moderna vaccine to allow deployment of more doses per vial, officials announced. It is authorized for use in people aged twelve years and older in some jurisdictions and. Refrigerator between 2°c and 8°c (36°f and 46°f) for up to 30 days prior to its first use.
Moderna is in talks with the food and drug administration about a proposal to put as many as 15 doses in each vial of the company's coronavirus vaccine — up from the current 10 moderna. 2 hrs 10 hrs 4 01 2021 5 01 2021.

0 Komentar